| Company Name | Contact Info | Location | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
LİGAMED TIBBİ MALZEMELER SANAYİ VE TİCARET LİMİTED ŞİRKETİ
KEÇİLİKÖY OSB MAH. İSMAİL KAHRAMAN CAD. NO: 1 İÇ KAPI: 1A YUNUSEMRE / / MANİSA / TÜRKİYE
|
Contact Info: +90 236 213 1014
|
Hall: 8
Stand: 817B
|
||||||||||||
| Product Groups | ||||||||||||||
|
||||||||||||||
- Company Info
- Products
- Represented Companies
- Company Brands
High-Quality, State-of-Art Technology Products, Reliable Service, Unconditional Satisfaction... Ligamed; has been founded in the health sector based on idealistic building blocks rather than conventional ones by bringing in modifications to its adopted missions around the main themes of high-quality, state-of-art technology products, reliable service, and unconditional satisfaction in order to represent our country in the domestic and international market. Ligamed Tıbbi Malzemeler Sanayi ve Ticaret Limited Şirketi (Ligamed Medical Devices Industry and Trade Limited Company) designs, manufactures, and sells spinal surgery implants and has the following products in its product portfolio under the trademark of BIOMECH ; including the Thoracolumbar Stabilization System, Cervical Cage System, Lumbar Cage System, Corpectomy Cage System, Anterior Cervical Plate System, Posterior Cervical Screw System, Ligamed keeps its pace in the investments in the fields of Orthopaedic Surgery Implants and Dental Implants. Our company ; aims to provide the best service in this sector by developing steadily with its expert and qualified staff ensuring customer satisfaction, respecting patient rights, and exchanging information with the healthcare professionals in collaboration.

Polyaxial - Monoaxial - Spondylolisthesis Polyaxial screws and screws for spondylolisthesis allow for screw angulations of up to ±27,22°. The pedicle screws are more resistant to abrasions owing to their double grooved and double diameter features. Furthermore, it allows the user to achieve a faster and more controlled implantation. The screws are flexible enough for the implantation to be performed at any location. The screws are colour-coded to allow for differentiating between screw sizes easily. A specific thread structure is used for increasing the compatibility between the body of the screw and the locking screw. Thus; the pedicle screw - the rod - the locking screw are enabled to grip more safely.

Cervical Titanium Cage is manufactured by using Titanium Ti6Al4V-ELI Grade 5 material, which is compatible with MRI and CT and which does not result in permanent lesions. This product is implanted at the anterior disc distance by using the Smith-Robinson technique. Owing to its threaded surface feature; which allows for holding to superior and inferior surfaces, implantation can be completed without a need for fixation with a second implant such as a cervical plate. The availability of trial implants of various sizes used for selecting the appropriate size of the product and the potential of performing the implantation procedure by using a Biomech Trial Inserter as the only hand-held tool offer the user a practical and a time saving procedure. Advantages The parts of the cages in contact with the corpi are threaded, allowing for an easy grip. The cages are perforated and designed to allow for filling with bone grafts. The broad area of the cage surface contacts the corpus preventing collapses. Indications Disc herniation Degenerative discopathy and instability Restoration of the disc height
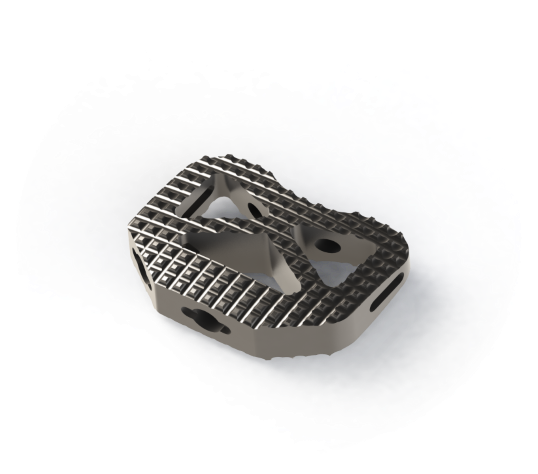
Biomech Lumbar Peek Cage is manufactured from MRI and CT-compatible PEEK material, which does not result in permanent lesions. PLIF is particularly useful for the elimination of neurological compression and for the correction of degenerative lumbar deformities such as spondylolisthesis, scoliosis, or disc space collapse. It has a serrated structure that allows for clinging to the superior and inferior surfaces. The proximal and distal surfaces of the cage are designed in an inverse v-woven pattern to prevent its dislocation. Adequate spacing over the cage allows for achieving the intended fusion by using the grafting technique. In this product group, two different PLIF Cages are available. PLIF Cage Expandable PLIF Cage Indications Disc herniation Degenerative discopathy and instability Restoration of the disc height General Features of the Products Anatomical design allowing for proper fitting to the endplates Evenly distribution of the load Restoration of lordosis Serrated surface Titanium marking rod A 2°-inclination in the design for anatomical fit Operational efficiency and flexibility Easy and convenient use Proper interior design for grafting Surgical Technique 1.The patient is positioned appropriately depending on the pathological condition and to allow for performing the Smith Robinson technique. After starting monitorization of the patient, placing the cannulas, and administering anaesthesia; the patient is brought to the supine position. Care should be exercised about the potential pressure points and the back of the patient during positioning. Consequently, the surgical site is prepared by using sterile covers and drapes in compliance with the general procedures. 2.Skin and subcutaneous tissues are incised at the surgical site. The skin and subcutaneous tissue are incised via a hemi-collar skin incision (on the right side of the patient). The platysma is incised vertically. Thus, the prevertebral fascia is exposed. 3. The space is examined by using a C-arm scopy (mobile C-arm X-ray unit). Discectomy is performed to clear the space around. The osteophytes and disk fragments are removed by using a curette. 4.Of different sizes of BIOMECH Interbody Lumbar Prosthesis, the appropriate one for the intended surgical site is selected and implanted under C-arm scopy. 5.Consequently, the surgical site is closed with conventional methods.
■ %100 Synthetic Contains no tissue of human or animal origin therefore carries no risk of disease transmission. ■ Osteoconductive Act as a scaffold and support bone tissue regeneration. Similar to the mineral found in bone tissue. Powerbone polygonal granules have different particle sizes between 0.25-9 mm. ■ Bioresorbable With its optimized porous structure and chemical composition, Ligamed is suitable for the continuous remodeling cycle of healthy bone. ß-TCP resorbs over time and be replaced with bone during the healing process. ■ Safe, Biocompatible and Sterile Powerbone is supplied sterile and CE marked as a Class III Medical Device according to Directive 93/42/EEC. Ligamed is tested using: Pre-clinical studies, Biocompatibility tests (in vitro and in vivo). Biomechanical tests, Biodegradation tests, Bioburden and Sterility tests. ■ Radiopaque Could be detected via CT and X-ray.
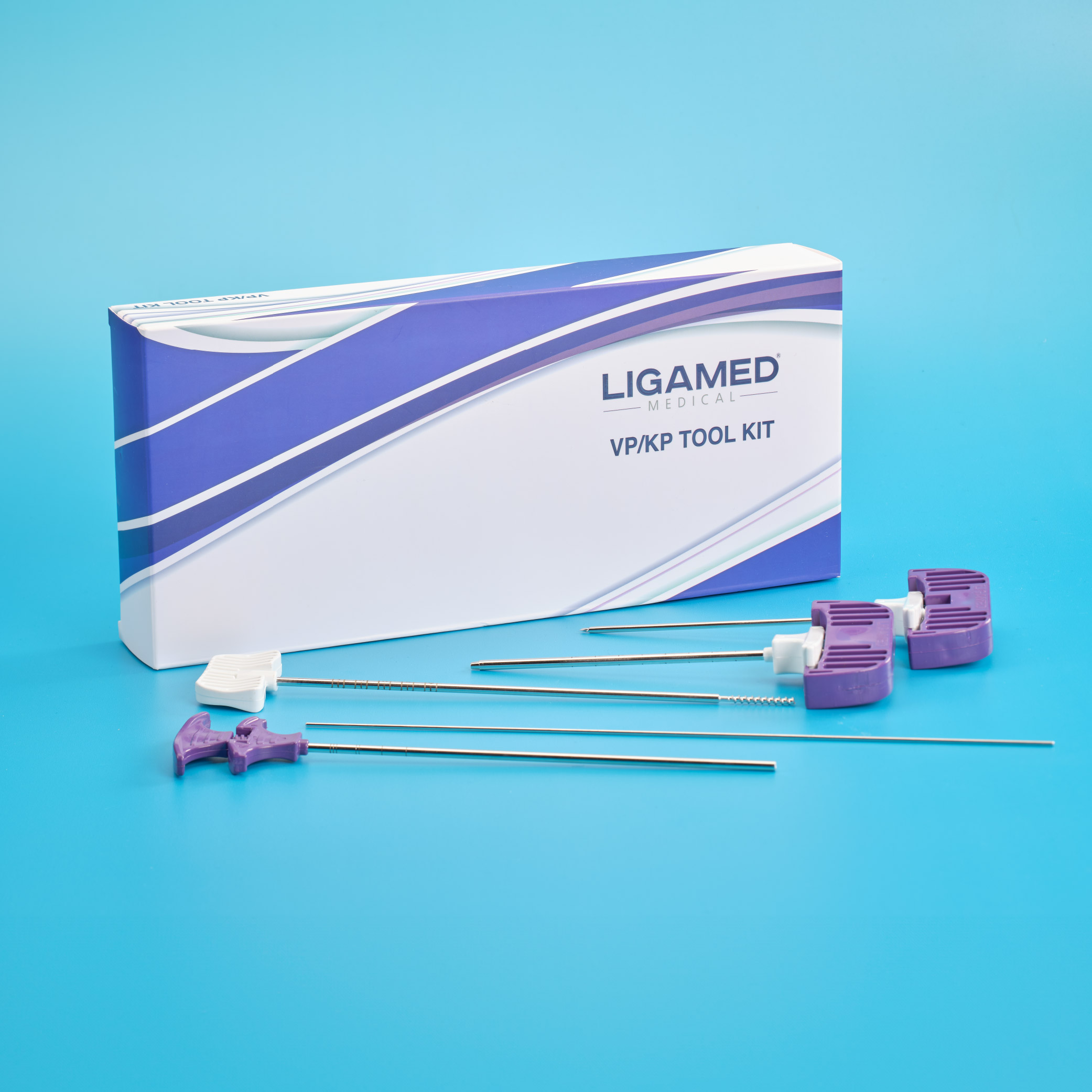
Bone Cement based on polymethyl methacrylate (PMMA) is a widely used biomaterial due to its ease of use in clinical practice and especially the long survival rate proven by dentures. ■ Common Indications for Bone Cement: total joint replacement are bone and joint reconstructions, fracture fixation and treatment of osteoporotic vertebral fractures. ■ Bone Cement consists of two phases, solid and liquid phase. ■ To use the product, two phases are mixed in the mixing bowl for 30 seconds. ■ Since the product is offered in three different viscosities, low, standard and high, it can be used in different surgical applications at Spine&Ortho. ■ Products have paste, hardening, maximum temperature and mechanical strength values specified in ISO 5833 standard.
No represented companies found.
- BİOMECH

 TR
TR
