| Company Name | Contact Info | Location | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ERASER MEDİKAL TIBBİ CİHAZ. SAĞLIK ÜR. PAZ. SAN. VE TİC. LTD. ŞTİ.
Kemalpaşa Mah. Pınar Cad. No: 78 Pınarbaşı / Bornova / İZMİR / TÜRKİYE
|
Contact Info: +90 232 479 8060
|
Hall: 3
Stand: 334B
|
||||||||||||
| Product Groups | ||||||||||||||
|
||||||||||||||
- Company Info
- Products
- Represented Companies
- Company Brands
Eraser Medical LLC was founded in 2007 in İzmir. It started its services in the fields of preparation and administration of chemotherapy and TPN mixtures with the experience and knowledge gained by its expert staff as a result of many years in the sector. The company registered its experience with the ONCOERA brand, which it has been producing since 2014. Today, Eraser Medical is the manufacturer, authorized dealer and exporter of ONCOERA, NUTRIERA, ONCOMIX and ERAVERSE branded products. Eraser Medical LLC, which produces disposable sterile and non-sterile medical devices and medical supplies, also manufactures Chemotherapy, TPN, Aseptic Drug Preparation Devices and software, by its strong R&D infrastructure. Company has adopted a scientific-based and customer-oriented production and sales policy. Products of Eraser Medical are exported to 4 continents. Production Facility The production facility has a closed area of 1,700 m2. The production, assembly and packaging of medical devices is carried out in 2 Clean Rooms of Class 10,000 and 2 Clean Rooms of Class 100,000. The biological load on the products is kept under control in these clean rooms and sterilization safety is ensured. Disposable medical equipment production consists of injection, extrusion, assembly, packaging and sterilization sections. The storage processes of the products before loading are monitored in a 500 m2 area. The raw materials used in production are biocompatible and meet USP and ISO 10933 standards. Eraser Medical LLC has ISO 9001, ISO 13485 and CE certificates. In the production facility, ONCOERA, NUTRIERA, ONCOMIX and ERAVERSE branded products registered to Eraser Medical LLC are produced. R&D Center The R&D center is structured to develop software and device hardware and has a wide range of device park. R&D processes are advanced by channeling the years of experience of the marketing, engineering, software, medical and quality teams to a common point in order to develop technology. In research and development activities, both the needs of the sector are taken into account and the most up-to-date scientific trends in the field of health are utilized.

ONCOERA® Vial Adapters are equipped with a 0.2 µm hydrophobic air filter to protect users from aerosols and drugs from microbiological contamination. It creates a closed system when used with syringe adapters.

ONCOERA® LINE Oncology Transfer Sets are extension lines with needle-free connectors, which allow the connection of prepared drugs to the administration sets and ensure the continuity of the closed system. Transfer sets may include an inline filter or a needle-free connector to meet different requirements. It can be designed with or without UV protection and set lengths can be configured as desired. Supports closed system. Protects compounded drug, personnel, and compounding environment. UV protected set options are available for drugs that require UV protection. Set options with 0.22 micron filters are available for drugs containing Paclitaxel. Integrated valve options are available to prevent free flow. DEHP-Free

Antineoplastic drugs used in cancer treatment should be prepared, administered and disposed under special conditions due to their toxic effects. Administration of the compounded drugs at the right speed and at the right dose is extremely important for the effectiveness of the treatment. ONCOERA® Oncology Infusion Sets are the safest solution used in chemotherapy administration and prioritizing patient comfort. Sets contain needle-free connectors that ensure the continuity of the closed system. Supports closed system. Protects compounded drug, personnel, and environment. UV protected set options are available. Set options with 0.22 micron filters are available, for drugs containing Paclitaxel. Integrated valve options are available to prevent free flow. Removes particles from the system with a 15 µm particle filter in the drip chamber. Multiple infusions of fluids can be done quickly and safely through a single set. Reduces the workload significantly and saves time and cost. DEHP-Free
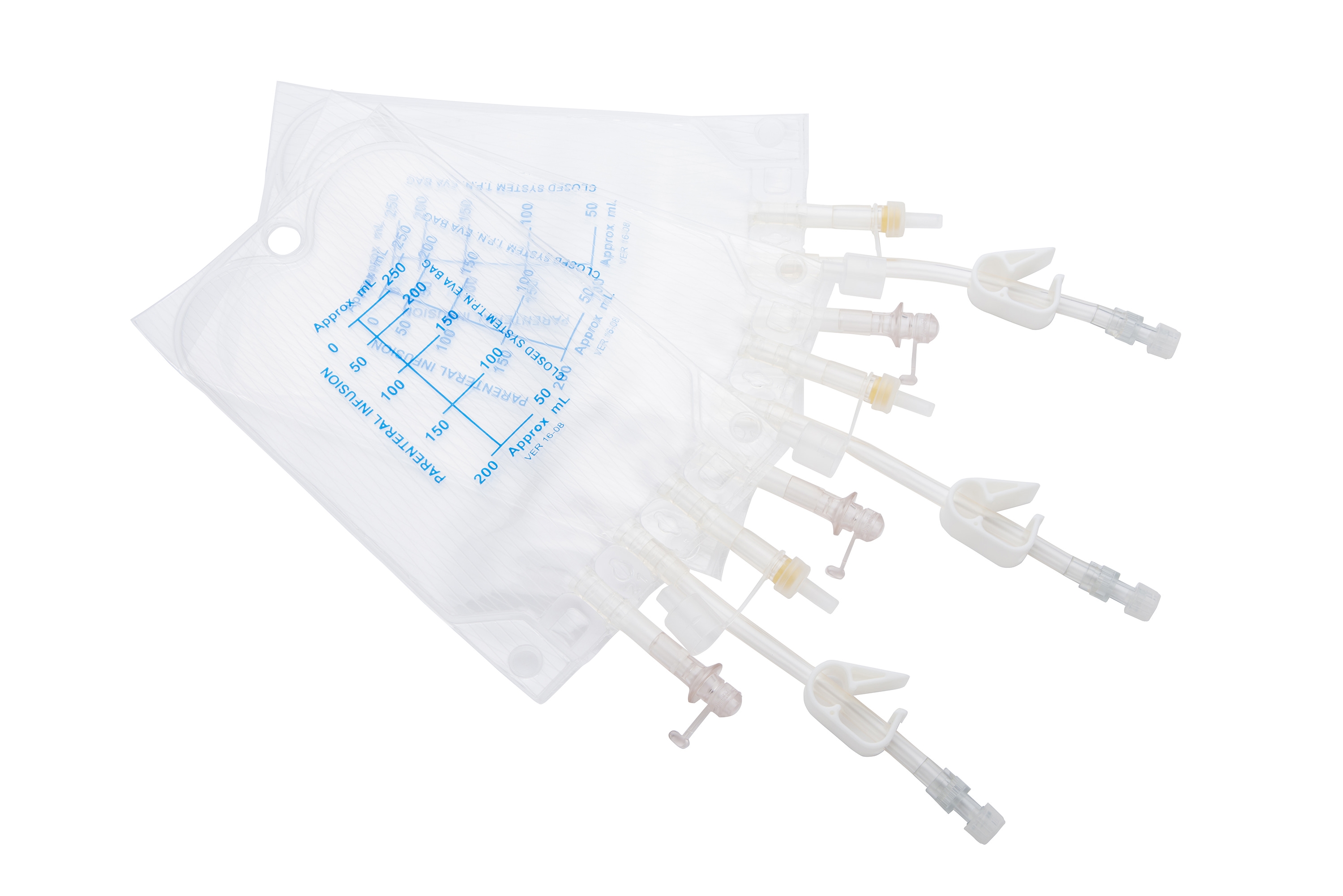
Ethylene vinyl acetate bags with male or female luer ends or Big Bore Ports. UV protected or unprotected options are available. Available in different volumes from 250 mL to 5,000 mL. Bag clamps are designed as openable or non-openable on demand. DEHP and LATEX free. Suitable to use in automatic compounders. UV protected options are available. 250 - 500 1,000 2,000 3,000 5,000mL bag options are available.
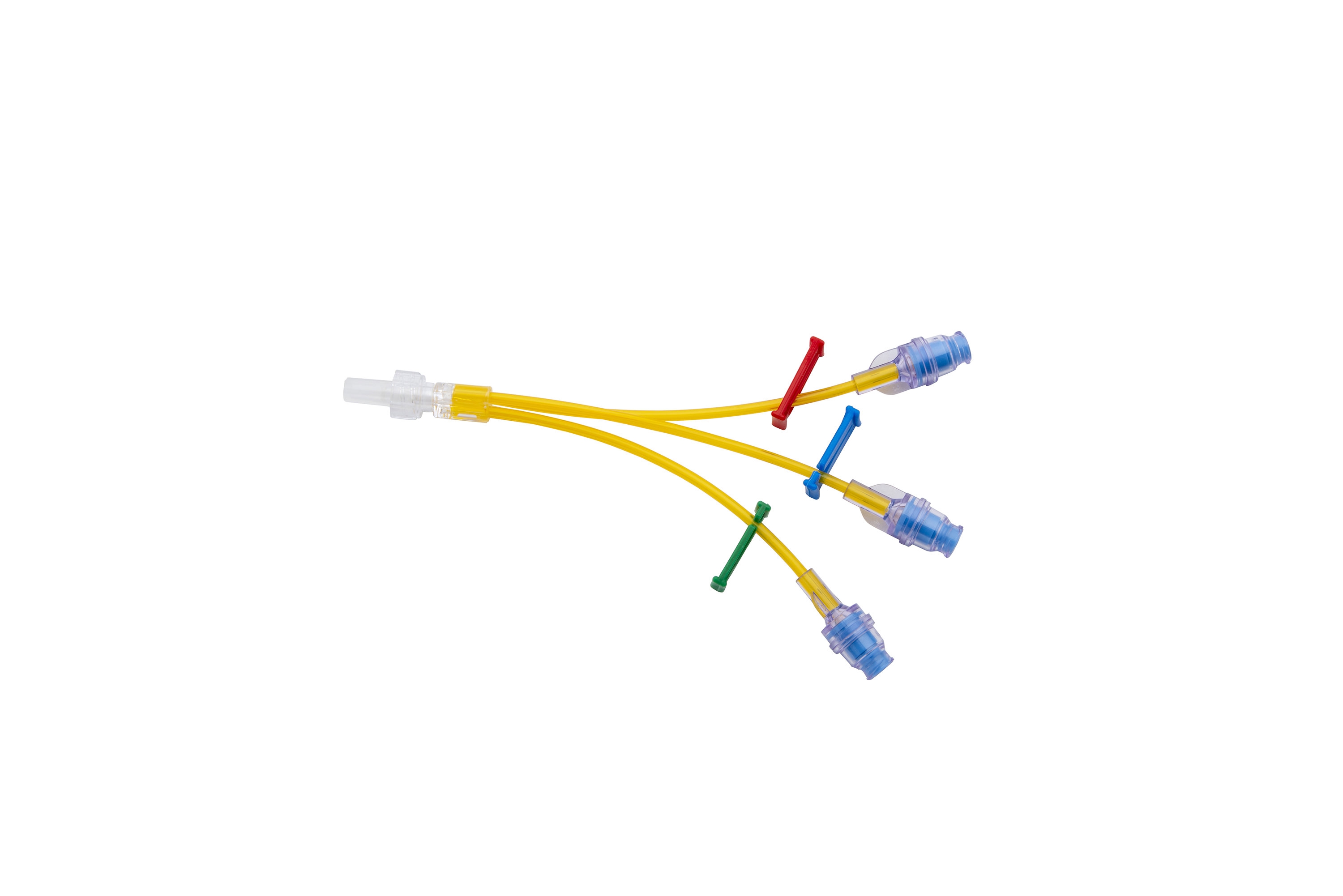
ONCOERA brand provides to its customers extension lines with different lenghts and diameter options with variety of accessories , needleless connectors , bag spike and etc.
No represented companies found.
- ONCOERA
- ERASVERSE
- ONCOMIX
- NUTRIERA

 TR
TR
